Daybue (trofinetide) for Rett syndrome
Last updated Oct. 9, 2025, by Marisa Wexler, MS

What is Daybue for Rett syndrome?
Daybue (trofinetide) is a daily treatment for people with Rett syndrome, ages 2 and older. It has been shown in trials to reduce the severity of Rett symptoms. The therapy, which is sold by Acadia Pharmaceuticals, can be taken by mouth or via a feeding tube.
The therapy’s active ingredient is an analog of insulin-like growth factor-1 (IGF1), a signaling molecule that helps regulate the development of brain cells. It’s thought that Daybue may help nerve cells form healthier connections, or synapses, and reduce inflammation in the brain.
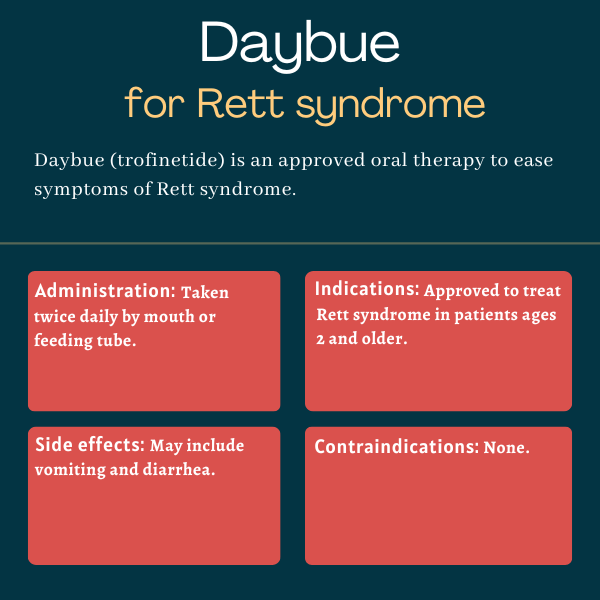
Therapy snapshot
| Brand name: | Daybue |
| Chemical name: | Trofinetide |
| Usage: | Used to reduce the severity of Rett symptoms |
| Administration: | Taken orally, twice daily |
Who can take Daybue?
Daybue is approved by the U.S. Food and Drug Administration (FDA) to treat people with Rett syndrome ages 2 and older. It is similarly approved in Canada.
The prescribing information for Daybue does not list any contraindications.
How is Daybue administered?
Daybue is a liquid that may be taken orally or administered by a feeding tube. It is taken twice daily at a dosage determined based on the patient’s weight. The therapy may be taken with or without food.
Daybue in clinical trials
The FDA’s approval of Daybue was based on data from a Phase 3 clinical trial called LAVENDER (NCT04181723). The study tested the therapy against a placebo in 187 people, ages 5 to 20, with Rett syndrome.
- The results showed that, after 12 weeks, or about three months, patients given Daybue had significantly greater improvements on the Rett Syndrome Behaviour Questionnaire (RSBQ), a caregiver-rated assessment of the severity of Rett symptoms. The RSBQ measures symptoms such as repetitive hand movements and facial expressions. Significantly greater improvements were also seen on the Clinical Global Impression Scale-Improvement (CGI-I), a clinician-rated measure of overall health.
Some participants who completed the LAVENDER study continued to receive Daybue in the extension studies LILAC-1 (NCT04279314) and LILAC-2 (NCT04776746). Long-term data from these studies indicated that RSBQ and CGI-I scores tended to continue improving over several years of Daybue treatment.
A Phase 2/3 study called DAFFODIL (NCT04988867) tested Daybue in 15 children with Rett syndrome, ages 2 through 4. Those results indicated that the treatment led to improvements in CGI-I scores and measures of life quality.
Daybue side effects
The most common side effects associated with Daybue in clinical trials were vomiting and diarrhea.
Patients who experience severe diarrhea, vomiting, or weight loss may need to pause treatment, reduce their dose, and/or discontinue the therapy. The use of laxatives should be stopped before starting Daybue.
Rett Syndrome News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- New study links Rett energy problems to specific gene mutations
- A Rett syndrome clinical trial was our way of paying it forward
- Partners to design AI-based gene editing therapies for Rett
- Rett gene therapy trial starts dosing patients
- Iron-fueled death of cells may drive Rett syndrome damage: Study
- How Rett syndrome affected my children’s birth order traits
- Rett syndrome twins hint at hidden genetics in disease severity
- Taysha regains full rights to TSHA-102 gene therapy for Rett
- I lost 40 pounds — twice
- Pivotal NGN-401 trial speeds toward dosing for people with Rett
