Daybue (trofinetide) for Rett syndrome
What is Daybue for Rett syndrome?
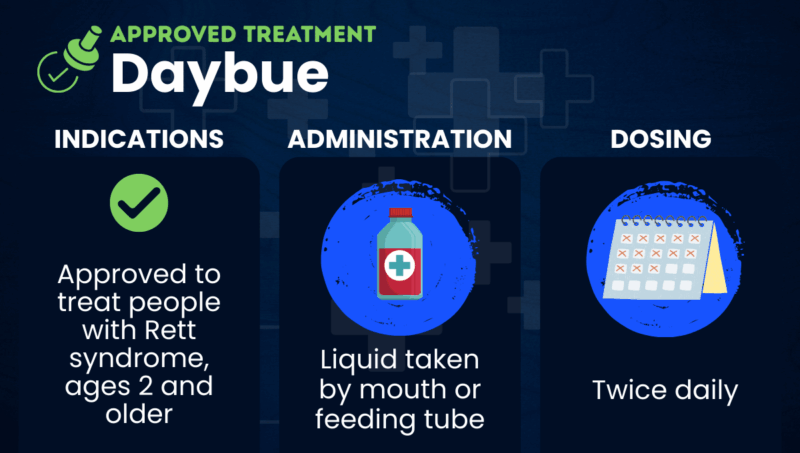
Daybue (trofinetide) is a daily treatment from Acadia Pharmaceuticals that’s approved for people ages 2 and older with Rett syndrome. The therapy, taken by mouth or via a feeding tube, has been shown in clinical trials to reduce the severity of Rett symptoms.
Rett syndrome is a genetic disorder that causes problems with brain development, leading to a wide range of possible symptoms.
Daybue’s active ingredient is a lab-made version of insulin-like growth factor-1, a signaling molecule that helps regulate the development of brain cells. Scientists don’t understand exactly how the medication works, but it’s thought to help nerve cells form healthier connections, or synapses, and support the brain’s ability to adapt and learn. Treatment with Daybue is also thought to reduce the inflammation that contributes to neuronal dysfunction in Rett.
Daybue is available as a ready-to-use liquid. The medication also comes in a dye- and preservative-free powder formulation, called Daybue Stix, that must be dissolved in liquid before administration.
While the two formulations are expected to have essentially identical effects in the body, Daybue Stix is designed to be a more customizable option, allowing patients and caregivers to make choices regarding dose volume and taste.
Therapy snapshot
| Brand name | Daybue and Daybue Stix |
| Chemical name | Trofinetide |
| Usage | Used to reduce the severity of Rett symptoms |
| Administration | Liquid taken by mouth or feeding tube, twice daily |
Who can take Daybue?
Daybue and Daybue Stix are approved in the U.S. for treating adults and children, ages 2 and older, with Rett syndrome. The liquid Daybue formulation is similarly approved in Canada.
The prescribing information for Daybue does not list any contraindications for its use. The medication is generally not recommended for people with severe kidney impairment.
How is Daybue administered?
Both Daybue and Daybue Stix can be administered orally or via a feeding tube. They are typically taken twice per day, with or without food, and are strawberry-flavored. The recommended dose depends on the patient’s weight, ranging from 5,000 to 12,000 mg twice daily.
- Daybue comes as a liquid. The appropriate dose should be measured out using a device provided by a pharmacy.
- Daybue Stix comes in packets of powder that must be completely dissolved, immediately before use, in cold or room-temperature water, or water-based beverages such as juice, tea, lemonade/limeade, or a hydration drink.
Depending on the dose, one or more packets of Daybue Stix may be required; the volume of liquid needed to completely dissolve the medication varies according to the dose. The specific volume within the recommended range is selected based on individual factors, such as taste preferences.
The dose of Daybue or Daybue Stix may need to be modified for people with moderate kidney impairments, or if severe diarrhea, vomiting, weight loss, or suspected dehydration occurs.
Daybue in clinical trials
Daybue’s U.S. approval was based on data from a Phase 3 clinical trial called LAVENDER (NCT04181723), which tested the therapy against a placebo in 187 people, ages 5 to 20, with Rett syndrome. After 12 weeks, or about three months, of treatment, participants who had received Daybue showed significantly greater improvements than the placebo group in:
- caregiver-rated symptom severity, measured with the Rett Syndrome Behaviour Questionnaire (RSBQ), which assesses key Rett symptoms such as repetitive hand movements and facial expressions
- clinician-rated overall health, measured with the Clinical Global Impression Scale-Improvement (CGI-I)
- caregiver-rated communication ability
Some participants who completed LAVENDER continued to receive Daybue in the extension studies LILAC-1 (NCT04279314) and LILAC-2 (NCT04776746). Long-term results from LILAC-1 and LILAC-2 indicated that RSBQ and CGI-I scores tended to continue improving over several years with Daybue treatment.
A separate Phase 2/3 study called DAFFODIL (NCT04988867) tested Daybue in 15 children, ages 2 to 4, with Rett syndrome. Although the study was small, those results suggested that the treatment led to improvements in CGI-I scores and measures of life quality in this younger population.
The approval of Daybue Stix was based on a clinical bioequivalence study, which demonstrated that the new formulation results in the same exposure to the active ingredient as the original formulation. This indicates that the same safety and efficacy can be expected from both versions of the medication.
Daybue side effects
The most common side effects associated with Daybue in clinical trials were vomiting and diarrhea. Given that most people who use the medication experience diarrhea, patients should stop using laxatives before starting Daybue or Daybue Stix.
Daybue’s prescribing label comes with warnings that it could cause diarrhea, vomiting, or weight loss that, in some cases, may be severe or significant. Vomiting after Daybue could lead to aspiration — when stomach contents accidentally enter the airways — which can result in a lung infection called aspiration pneumonia.
Patients or caregivers should notify a healthcare provider if any of these side effects occur so that appropriate treatment can be administered. In severe cases, patients may need to pause treatment, reduce their dose, or discontinue the therapy altogether.
Daybue may interact with other drugs. Healthcare providers should be notified of all other medications a patient is using.
Rett Syndrome News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
 Fact-checked by
Fact-checked by